Randall Anderson was 9 years old when his knees started swelling like soda cans left too long in the freezer. Fatigue knocked him flat for hours at a time. Red rashes ravaged his skin. Finally, the headaches began, the screams, the scans showing signs of brain inflammation. The adults didn’t know what was wrong with him. His elementary school worried that he might be carrying some contagion and sent him home with a private tutor for a month. Some nights during the ordeal he would sleep on his parents’ bedroom floor and squeeze his mother’s hand, afraid he wouldn’t make it to morning.
This was in the mid-1970s, in the little town of Old Lyme, Conn., a wooded enclave near the north shore of Long Island Sound, where active kids like Anderson could romp for hours in the gentle New England forest with little fear. Only later, after years in and out of clinics where doctors drained warm yellow liquid from his knees, did the true details of the disease that afflicted Anderson emerge. He had been infected by a corkscrew-like bacterium, a wily spirochete that we know today as Borrelia burgdorferi. Somewhere in the woods outside his home, a black-legged tick had injected the infectious organisms into his bloodstream. Anderson—who asked that his real name not be used in this article to protect his privacy—was one of the kids in that early cluster of childhood cases in the town that would give the disease its infamous name.
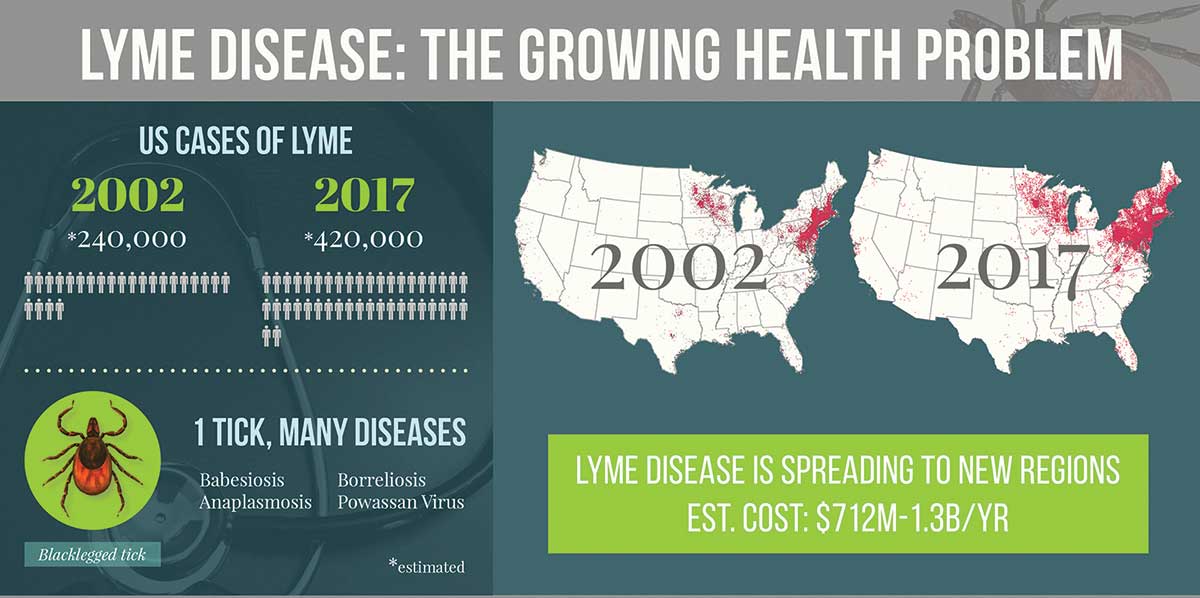
Lyme disease today, though less of a mystery, remains confounding, and it is spreading, sometimes to devastating effect. From that outbreak in New England, as well as a smattering of early cases elsewhere around the country, Lyme has been unrelenting in its march across the eastern United States and the Midwest. Carried by the black-legged tick (often referred to as the deer tick), it is the most common vector-borne disease in the country. And its incidence is increasing, with some estimates placing the number of new cases as high as 476,000 each year.
“That is a huge number,” says Dr. Ben Beard, the deputy director of the CDC’s Division of Vector-Borne Diseases. “And that doesn’t even include all the other tick-borne illnesses,” he noted, including babesiosis, the Heartland virus disease, the Bourbon virus disease, anaplasmosis, Rocky Mountain spotted fever, Colorado tick fever, Borrelia miyamotoi disease, and the deadly Powassan virus disease, which can cause encephalitis and has a case fatality rate of around 10 percent.
“For us,” he says, “that is very concerning.”
How did we get here? Why did Lyme disease emerge—or re-emerge—when it did? And why has it spread with such tenacity?
The short answer: heedless human meddling with Mother Earth. We pull at the strings of nature, and the consequences of its unraveling are impossible to reckon. Lyme, like Covid-19, is a zoonotic disease, which means it spills from animals into people. In Lyme’s case, the pathogen is passed to humans via a vector—specifically, by black-legged ticks, those sesame-seed-size arachnids with eight legs and anesthetizing bites. Like so many other zoonotic diseases, Lyme and other tick-borne pathogens have emerged from ecosystems that have been disturbed, fragmented, and fractured by intensive human development. Ballooning deer populations, second-growth forests, suburban and exurban growth, habitat degradation, predator eradication, wildlife extinction—these and other factors have set the stage for the surging presence of Lyme and its zoonotic cousins among us.
Popular
"swipe left below to view more authors"Swipe →
As a result, millions of Americans now live in a world where a simple walk in the woods may lay us low or even upend our lives. It’s a world in which ticks and the diseases they disgorge now seem as persistent and inevitable as a wave—moving, shifting, spreading to new places.
Borrelia burgdorferi
Though ticks were still rare in parts of the Northeast when Randall Anderson was a kid, they were common in Lyme. His house was surrounded on two sides by forest, and Anderson liked to bike in the woods, so the sight of minuscule arachnids crawling up his leg was hardly a novelty. “When I was a kid, I’d just pull them off and flush them,” he says. No biggie.
Then everything changed. In the summer of 1976, The New York Times ran a story: “A New Type of Arthritis Found in Lyme.” Documenting the dozens of children, including Anderson, who were experiencing painful swelling in their joints, the article reported that doctors and scientists believed they had discovered a new “form of arthritis caused by a virus carried by an insect or other biting arthropod such as a tick.”
One of the doctors involved in early work on the disease was Allen Steere, an infectious disease epidemiologist and rheumatologist at Yale who took care of Anderson and other sick children during the initial years of the outbreak. Steere was instrumental in tracing the illness to ticks. Others made key contributions as well, including Dr. Willy Burgdorfer, a researcher at the National Institute of Allergy and Infectious Diseases’ Rocky Mountain Lab, whose work helped show definitively that the disease was caused by a species of spirochete bacterium, a type that shares a distinctive shape with the pathogens that cause syphilis and tick-borne relapsing fever. Burgdorfer, after his team’s discovery, became the pathogen’s namesake. He died in 2014. (I should mention that some people believe that Lyme disease didn’t spill over naturally. Because Burgdorfer had ties to the US military, and because the US government’s Plum Island biowarfare and animal research lab was nearby in Long Island Sound, they speculate that the emergence of Lyme was the result of a lab leak, some sort of government bioweapons program gone wrong. Ross Douthat usefully delves into this theory in his memoir The Deep Places. The scientists I asked about this theory discounted it.)
While Lyme disease was greeted as a novel phenomenon, there is now plenty of evidence indicating that Borrelia burgdorferi has been on this continent in various forms for thousands of years (as it has been in Europe). According to a 2017 study in the journal Nature, Ecology, and Evolution in which scientists sequenced nearly 150 different Borrelia burgdorferi genomes, the bacteria are “ancient.” They have been evolving in North America for roughly 60,000 years and were historically widespread across the Northeast and Midwest.
“Our finding of ancient B. burgdorferi diversification suggests that the recent Lyme disease epidemic does not reflect evolutionary processes,” the scientists wrote, “but rather was driven by the ecological change in North America beginning in the colonial period [roughly] 700 years ago.”
If the Lyme pathogen has been here all along, why was the disease absent—or at least not rampant—during this country’s first two centuries? And why has it returned in such a ferocious fashion?
“Ecological change,” as the scientists say above, is a polite way to put it. “We fucked up the ecosystem” is probably a better way to say it.
Black-legged ticks—and here I am referring to Ixodes scapularis, the species that lives among us here in the East; a similar species, Ixodes pacificus, also a Lyme carrier, is confined to pockets of the West Coast—are primarily forest creatures. These ticks need forests to survive and thrive in most of their range, which sits on the humid side of the 100th meridian. But beginning in the colonial period, the eastern forests fell rapidly as European settlers cut, burned, and girdled their way through the vast stands of oak, hemlock, chestnut, and other species that grew along the Atlantic littoral. By the middle of the 19th century, roughly three-fourths of southern New England had been deforested, transformed into fenced pastures, farm fields, and settlements, as William Cronon recounts in his classic book Changes in the Land. What’s more, colonists rendered large areas of New England “devoid of animals which had once been common: beaver, deer, bear, turkey, wolf and others had vanished.”
The enormous destruction visited on the Northeast’s forests wiped out the black-legged tick’s habitat in many places. And without black-legged ticks, Borrelia burgdorferi cannot persist. Hence the apparently scant presence of Lyme disease during the first 150 years and more of this country’s history. But that began to change after the completion of the Erie Canal, as farmers and pastoralists left the Northeast’s depleted lands for the rich post-glacial soil of the Midwest. The forests came back in a big way. By the mid-20th century, roughly 60 percent of Connecticut had returned to forest. Likewise in New York: Many once-denuded landscapes are now gorgeous with deciduous groves.
But the second-growth forests we live with today are not the same as those that preceded European colonization and conquest. The forests are back, but without the low-severity forest fires that Indigenous people used to burn away underbrush, possibly reducing tick populations. The oak trees too are back, but without the sky-darkening droves of passenger pigeons that once competed with rodents for tree nuts and possibly suppressed mouse and chipmunk abundance in the process. White-tailed deer are also back, but the wolves and cougars that helped keep their populations in check are still consigned to oblivion. The forests, in other words, are missing many ecological influences that may have played a role in regulating the various components of the Lyme disease system.
“The forest creates the stage on which the actors occur,” one scientist told me.
By the second half of the last century, the stage for the Lyme disease drama was set. It was one that was particularly well-suited for every phase of a tick’s life.
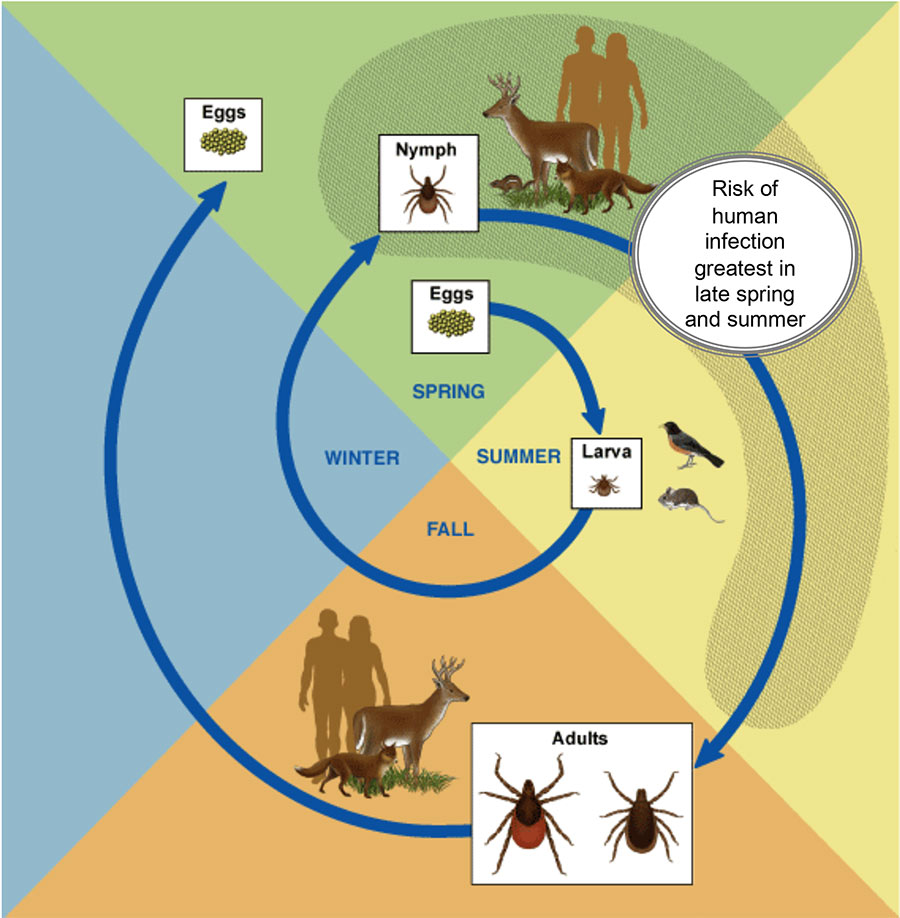
Larva
In a forest of maple, oak, and pine on the eastern side of New York’s Hudson Valley, Rick Ostfeld and his team of research scientists march through the leafy undergrowth in bright white body suits worn for protection. They climb a small ridge and arrive at a forest plot studded with small metal traps. It’s early morning, and some of the traps, baited overnight with oats, are snapped shut. The team’s senior research specialist, Kelly Oggenfuss, opens one and reaches inside to reveal a small rodent, shivering, docile, its black eyes wide with fear. It seems harmless enough, poor thing; in fact, the trembling animal in her hand is a star player in the drama of Lyme disease.
White-footed mice, along with other forest rodents like chipmunks, are what scientists call reservoir hosts. They are the long-term hosts of the Lyme pathogen, the ultimate source from which the little corkscrew bacteria sally forth to infect new organisms. Oggenfuss points to the mouse’s paper-thin ears. Embedded in them are several minute black dots, no bigger than a pencil point: larvae, the first life stage of the black-legged tick.
The baby ticks are enjoying their inaugural “blood meal” at our rodent friend’s expense (female ticks will consume a total of three blood meals over the course of their lives, while males require only two). If this mouse is carrying the Lyme pathogen—and as many as 90 percent of the adult mice around here are carriers—then these larvae will grow into infected nymphs. By early next summer, as they search for a second blood meal to fuel their nymphal life stage, these ticks will pose a danger to human health. They might also go on to infect a new crop of mice with the Lyme pathogen, thus ensuring its continued presence in the reservoir host population. After that, in their third and final form, the ticks will be full-fledged adults, and the females will seek out one last blood meal, often from white-tailed deer, before reproducing. A single female tick can give birth to thousands of healthy offspring.
To summarize and simplify: Larvae pick up the Lyme pathogen from blood meal No. 1. Nymphs spread it readily during blood meal No. 2. Female adults reproduce during blood meal No. 3 and then die. Three life stages over about two years.
Ostfeld, a veteran scientist with hazel eyes and an earnest smile, has been studying the relationships between ticks, rodents, deer, other mammals, and their forest habitat for more than 30 years. A disease ecologist at the Cary Institute of Ecosystem Studies in upstate New York, he and his team have uncovered some of the key dynamics in the Lyme disease system. Through long-term trapping and analysis of mouse and tick populations in the forests around their lab, they have discovered that infected nymph abundance is closely tied to white-footed mouse abundance, and white-footed mouse abundance is heavily influenced by the availability of acorns and other foods on the forest floor. During a “mast” year, when oak trees drop vast amounts of acorns to increase their chances of reproduction, rodent populations will devour these nutritious treats, and their populations will shoot up the following year. This provides tons of blood-sucking opportunities for baby ticks, many of which will contract pathogens, including Borrelia burgdorferi, from the mice. Two years following a mast event, the number of Lyme-infected nymphs in the forest will peak right around May, June, and early July, when humans are having fun in the forest.
In their studies, Ostfeld and his team have found that white-footed mice play a much greater role than do deer—long linked with Lyme disease in the public imagination—in determining the density of infected nymphs in their study areas, though deer do have an effect as well. And they have found that the presence in the landscape of a diverse community of midsize predators, including foxes, is associated with a reduction in the abundance of infected nymphs: More mid-level predators = fewer rodents = fewer infected nymphs. Finally, as David Quammen explains in detail in his book Spillover, Ostfeld has found that smaller forest patches—forests that have been cut up and fragmented—have a higher density of infected nymphs than larger, more intact forest patches.
“It is kind of a perfect storm,” Ostfeld tells me as we stand in the forest, occasionally intercepting the ticks climbing up our big white body suits. “We have inadvertently enhanced the proliferation of infected ticks and also increasingly encroach on those very areas that we have made more risky.”
Nymph
Allen Steere, the rheumatologist who treated Randall Anderson and those other childhood cases back in the ’70s, was among the earliest witnesses to this fateful shift in both tick and human populations. Since identifying that original cluster of 51 patients—39 children and 12 adults, all with a similar type of arthritis—he has gone on to publish more than 300 papers on Lyme disease, and from his current perch as a professor at Harvard, he still sees patients. Indeed, some years ago he consulted once again with Anderson, who was experiencing eye problems, perhaps due to the lingering effects of Lyme.
To help me understand how the town of Lyme came to be the epicenter of the first known outbreak, I called Steere up one day this winter. He took me back to the early days and described the landscape around Lyme and Old Lyme, where the pathogen spilled so readily into the human population.
“It is a lovely area, right at the mouth of the Connecticut River, where it flows into Long Island Sound, with estuary areas and lakes and rivers. And it is wooded, second-generation wooded growth, but it is also inhabited,” he says. “So it was a perfect environment for deer and for ticks, and there were a lot of cases.”
It was a perfect environment for ticks—and it was inhabited. In the world of wildfire prevention and suppression, there is a concept called the wildland-urban interface, or WUI. It is the transition zone where wild land and human development mix and mingle, and it is often in such areas where wildfire poses the greatest risk to human life and property. Of course, people still build there anyway, erecting their homes amid California chaparral or thick stands of lodgepole pine, but it’s a big gamble.
One might think of the Lyme phenomenon in a similar fashion. It is in the wildland-urban interface in places like Connecticut and New York—where residential development meets forest—that human health is at heightened risk.
Consider Connecticut. By the 1970s, the degraded second-growth forests of New England, with their acorns, deer, mice, and ticks, had made a major recovery. At the same time, Connecticut had seen a huge surge in the human population, from about 900,000 in 1900 to more than 3 million by the 1970s. Many of these residents lived in homes like Anderson’s, nestled in the forests that had returned to the state. It was a WUI situation, but for Lyme disease. Indeed, across the country, since 1970, landscapes categorized as WUI have expanded by more than 50 percent as land conversion to exurban and suburban use has exploded. This trend has been particularly intense in the Northeast.
The scientific literature on zoonotic diseases suggests that their emergence is often driven by land-use changes like deforestation, residential and commercial development, and more. A 2015 paper in the journal Vector Borne and Zoonotic Diseases found that land-use change was the greatest driver of known zoonotic spillover events in recent decades. This finding fits well with the Lyme scenario in both the Northeast and the Midwest. Indeed, by the time Lyme had begun to spread widely, forest cover in places like Connecticut had hit its apex and was once again in decline as the human population blossomed.
“What exacerbates the risk is the fragmentation and degradation of that forest,” Ostfeld told me, “because that is how we facilitate some species’ population growth, especially the weedy little rodents that are so important in the proliferation of the ticks and the pathogens.” Rodents, deer, and other hardy habitat generalists do well in degraded forests that are often fragmented or perforated, low on predator and competitor diversity, and riddled with human developments. And it’s in partially fragmented landscapes that humans are at particular risk of encountering infected ticks.
You can see how this might play out. It’s early June, and you’re living in upstate New York or Connecticut or Long Island or Michigan or Wisconsin or Massachusetts. Maybe you go for a stroll in the woods near your suburb, maybe your dog runs after that squirrel bolting up an oak tree and then comes back to cuddle, maybe you’re just lounging in a sunny backyard abutting a patch of forest. A few days later you find an engorged tick in your armpit. It’s a nymph, much smaller than an adult and hard to see—the life stage during which the tick is most likely to pass the pathogen to humans. A year earlier, that nymph was a larva. It had spent some time sucking on the snout of a white-footed mouse, from which it contracted Borrelia burgdorferi. You pull the nymph out of your armpit, but maybe it’s too late. Nature isn’t always nice. The tick, in its single-minded quest for a second blood meal, has regurgitated the bacteria into your bloodstream. That’s how it happens. That’s how humans take their place on the stage.
Randall Anderson spent years battling Lyme disease. Since the doctors didn’t yet know what they were dealing with, it was a long time before he went on antibiotics. Eventually the sporadic brain inflammation stopped, but the swelling in his knees kept him out of gym class throughout middle school. By the time he was in high school, he says, it was mostly over.
“It just ran its course,” he says.
Adult
Meredith VanAcker stops outside the Staten Island Ferry terminal on a late May morning, scoops me up in her small Toyota, and together we embark on a brief tour of New York City’s tick problem.
A disease ecologist who recently completed her PhD at Columbia, VanAcker spent the past few years conducting field research into the dynamics of the Lyme disease system in urban settings. Specifically, she studied Lyme disease on Staten Island—because, yes, Borrelia burgdorferi is in New York City. Black-legged ticks are now firmly established on Staten Island and in the Bronx.
To understand why, VanAcker takes me to a place with an apt name: Deere Park. It’s a strip of forest surrounded by big single-family homes just south of Interstate 278, which cuts east-west across the northern part of the island. The first thing we see upon entering the park is the print of a deer hoof in the mud. Crucially, this park is one parcel in an interconnected complex of green space that extends to the western edge of the island, where a narrow channel of shallow water separates it from New Jersey. Deer, VanAcker tells me, can swim across that channel and as a result have managed to establish a robust presence on the island. In fact, their population here has grown so explosively in recent years that the city is leading a controversial sterilization effort to reduce their numbers. With the deer have come black-legged ticks, hitching rides like commuters on a ferry. And places like Deere Park, humid and shady with plentiful maples and oaks, provide excellent habitat not only for deer but also for mice, chipmunks, and similar creatures. Thus, Lyme disease has come to the island. Indeed, between 2000 and 2016, the case rate for Lyme on Staten Island has climbed from four per 100,000 to 25 per 100,000—a notable surge, although the case rate has dipped a bit in more recent years.
“Deer are so important for the [Lyme disease] system, but their role is important to distinguish,” VanAcker tells me. While they don’t host the Lyme pathogen and don’t pass it on to ticks, deer are critical “for distributing the ticks and amplifying the tick population,” she says. A single deer can “annually feed 500 adult ticks, which each will lay 2,000 eggs.” Deer, in other words, are key reproductive hosts for adult ticks. Without them, it is unlikely that ticks—and with them, Lyme disease—would have become established in a heavily urbanized environment like Staten Island.
To illustrate this last point, VanAcker takes me to another park in the borough, a place called Clove Lakes. This park is north of Interstate 278 and isolated from the snaking strip of green space that winds all the way to the island’s New Jersey–facing shoreline. Deer have a hard time crossing the highway, and as a result the tick population at Clove Lakes is scant.
“I wanted to bring you here because it has great habitat for tick populations…and the mice populations are really high here,” she says as we crunch through the forest understory. But the ticks are few and far between. “It is actually pretty low-risk [for Lyme disease], because [the park] is so cut off.”
But that’s not the case in many forest settings. As the number of deer has surged over the past century, amid the rise of hunting regulations and replenished habitat, they have become a key conduit for the spread of ticks in the Northeast and beyond. And with their high tolerance for human disturbance and penchant for chopped-up landscapes, backyard browsing, and the like, deer bring ticks into the places where people live, even urban areas.
“You are going to have ticks where you have people,” says Jean Tsao, a professor at Michigan State who has extensively studied the Lyme disease system in the Midwest. “[Deer] will bring ticks to the next subdivision—they are in the woods, the farm fields, and the neighborhoods.”
Deer, white-footed mice, disappearing predators, encroaching humans, fragmented forest—they all have a role in the Lyme disease system. And there’s another force at work, one that can never be ignored these days: climate change. “We have a good record of warming at the Cary Institute through our weather station here, and there has been a significant upward trend—it is warming here,” Ostfeld told me.
He and postdoc Taal Levi have found that tick larvae and nymphs are initiating their search for blood meals about a week and a half earlier each year because of the warming climate. That means ticks are increasingly active in early May, and humans should be prepared to protect themselves earlier than ever before. Climate change may also be contributing to the expanding range of black-legged ticks, especially to the north. And as Ostfeld has stated in interviews with other publications, it is likely doing the same for the white-footed mouse population, which is expanding its geographic range in the Midwest and in Canada, providing a host for the Lyme pathogen further north.
And then there are all those other diseases, all those other ticks, that are grabbing headlines of late. The lone star tick, once mostly confined to the South, seems to be becoming more prevalent in the Mid-Atlantic and the Northeast due to a warming climate. With it has come the alpha gal syndrome, which causes some people to become allergic to red meat. Scientists have also documented the emergence of a new invasive species, the Asian longhorn tick, which was first reported in the US in 2017. The females of this species can reproduce without mating, which means it can become incredibly abundant. In the US, these ticks have not yet been found to carry Lyme or similar diseases, but they could become a huge problem for livestock and wildlife. And the other diseases—anaplasmosis, babesiosis, Powassan virus, Rocky Mountain spotted fever, and more—they are still out there too, infecting people each year and with increasing frequency, though far less frequently than Lyme. What to do?
“It is an intractable, difficult problem,” Ostfeld says. “I am beginning to unfortunately come to the conclusion that there are going to have to be non-ecological solutions.” While he tries to stay optimistic, Ostfeld recognizes that preventing habitat fragmentation, keeping large forest ecosystems intact, getting deer, tick, and rodent populations under control, scaling back the WUI lifestyle that puts so many people at risk—all that can seem an insurmountable challenge in the United States today.
Ostfeld is putting his primary hope in vaccines. While an early vaccine, developed in the 1990s, was discontinued in 2002, scientists are again at work on vaccines that could protect people from the ravages of tick-borne illnesses. At Yale and other institutions, for instance, they are developing mRNA vaccines that hamper bacterial transmission and also trigger an intense skin reaction to tick bites themselves, alerting humans to the presence of the tick and allowing them time to pull it off before the little critter can inject its pathogens. It’s not particularly sexy. It won’t necessarily address the root cause of the problem. But that’s where things stand in the battle against these bloodsuckers. Upended ecosystems come with enduring costs.
“A perfect storm”—that is how Ostfeld described the rise of tick-borne disease in our time. Dr. Ben Beard of the CDC also used that phrase when we spoke on the phone. And finally, as I spent a sunny morning on Staten Island with Meredith VanAcker, I heard the apt, if careworn, phrase once again: “It’s like a perfect storm,” she said.